Heavy Metals Removal from Water by Efficient Adsorbents
Abstract
:1. Introduction
2. Heavy Metals Pollution
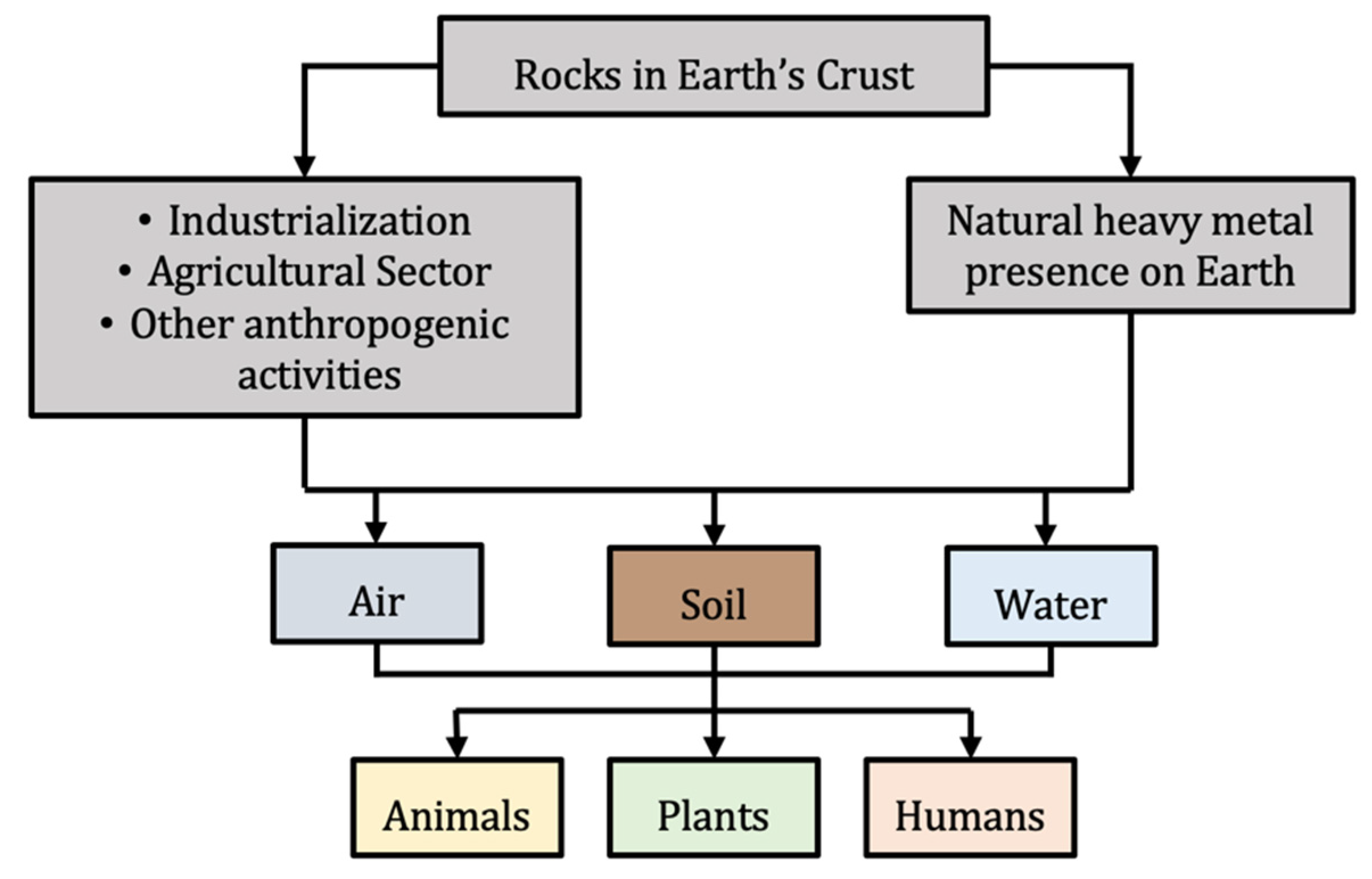
2.1. Sources, Toxicity and Risk of Heavy Metals
2.2. Heavy Metals Removal from Water
2.3. Heavy Metals Removing Methods
3. Remediation by Adsorption
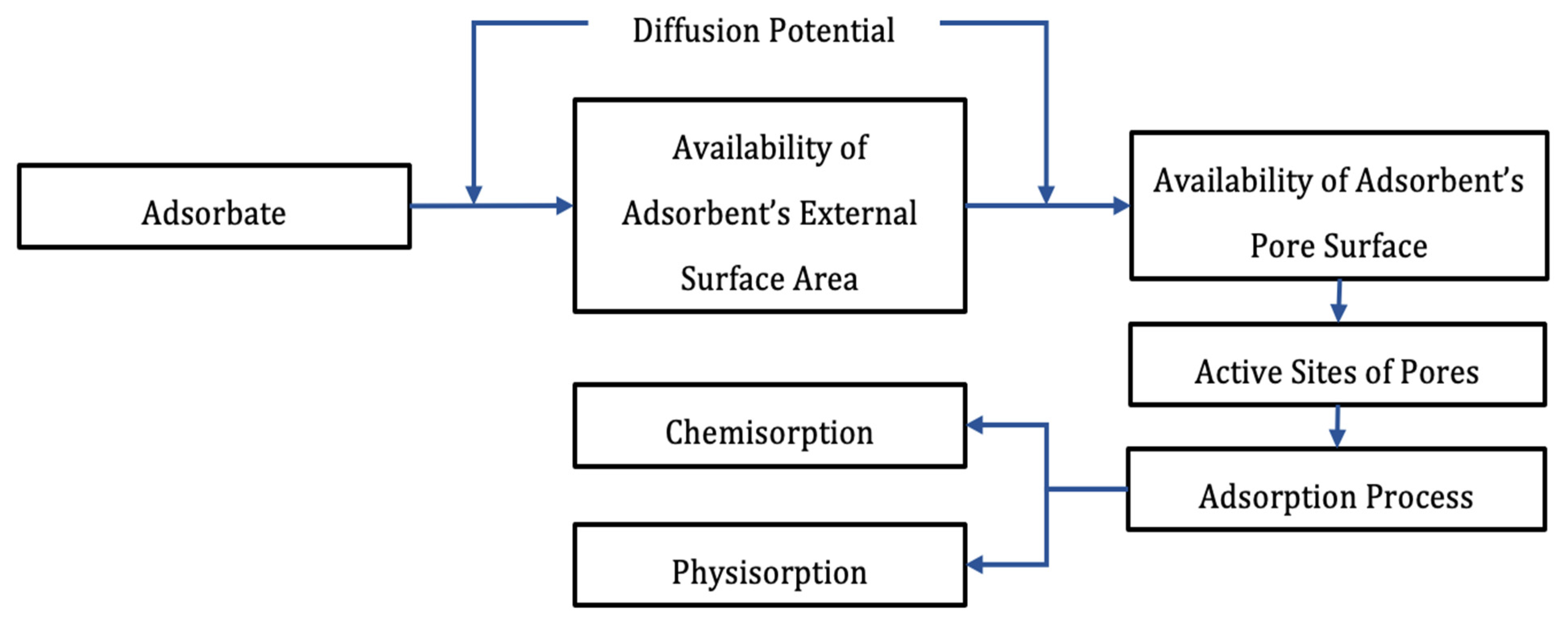
3.1. Overview of Adsorption Phenomenon
3.1.1. Classification of Adsorption
3.1.2. Factors Affecting Adsorption
3.2. Chelating Resins
Application of Chelating Resin in Removing Heavy Metals
3.3. Removal of Heavy Metals by Various Adsorbents
3.3.1. Polymeric Adsorbent
3.3.2. Industrial By-Product Adsorbent
3.3.3. Natural Mineral Based Adsorbent
3.4. Heavy Metals Removal Efficiency by Several Common Functionalized Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landrigan, P.J.; Fuller, R.; Fisher, S.; Suk, W.A.; Sly, P.; Chiles, T.C. Pollution and children’s health. Sci. Total Environ. 2019, 650, 2389–2394. [Google Scholar] [CrossRef]
- Ajibade, F.O.; Adelodun, B.; Lasisi, K.H.; Fadare, O.O.; Ajibade, T.F.; Nwogwu, N.A.; Sulaymon, I.D.; Ugya, A.Y.; Wang, H.C.; Wang, A. Environmental pollution and their socioeconomic impacts. Chapter 25—Environmental pollution and their socioeconomic impacts. In Microbe Mediated Remediation of Environmental Contaminants; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 321–354. [Google Scholar]
- Oves, M.; Khan, M.Z.; Ismail, I.M.I. Modern Age Environmental Problems and Their Remediation; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Sulaymon, I.D.; Mei, X.; Yang, S.; Chen, S.; Zhang, Y.; Hopke, P.K. Chemical Characterization, Source Apportionment, Temporal Variations, Transport Pathways and The Health Risks Assessment. Atmos. Res. 2020, 237, 104833. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.(N.); Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [Green Version]
- Jeevanantham, S.; Saravanan, A.; Hemavathy, R.V.; Kumar, P.S.; Yaashikaa, P.R.; Yuvaraj, D. Removal of toxic pollutants from water environment by phytoremediation: A survey on application and future prospects. Environ. Technol. Innov. 2019, 13, 264–276. [Google Scholar] [CrossRef]
- Abedin, M.A.; Shaw, R. Constraints and coping measures of coastal community toward safe drinking water scarcity in Southwestern Bangladesh. In Science and Technology in Disaster Risk Reduction in Asia: Potentials and Challenges; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated remediation processes toward heavy metal removal/recovery from various environments—A review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. Heavy Metals. 2018, 10, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Ewuzie, U.; Nnorom, I.C.; Eze, S.O. Lithium in drinking water sources in rural and urban communities in Southeastern Nigeria. Chemosphere 2020, 245, 125593. [Google Scholar] [CrossRef]
- Lutfor, M.R.; Mashitah, M.Y. Synthesis of poly(hydroxamic acid)-poly(amidoxime) chelating ligands for removal of metals from industrial wastewater. E-J. Chem. 2011, 8, 1038–1043. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Qin, G.; Zhang, Y.; Sun, J.; Wang, S.; Jiang, L. Heavy Metal Removal from Aqueous Solutions by Calcium Silicate Powder from Waste Coal Fly-Ash. J. Clean. Prod. 2018, 182, 776–782. [Google Scholar] [CrossRef]
- Sankhla, M.S.; Kumari, M.; Nandan, M.; Kumar, R.; Agrawal, P. Heavy Metals Contamination in Water and Their Hazardous Effect on Human Health—A Review. SSRN Electron. J. 2019, 5, 759–766. [Google Scholar]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from wastewater: A review. J. Water Process. Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Bagul, V.R.; Shinde, D.N.; Chavan, R.P.; Patil, C.L.; Pawar, R.K. New perspective on heavy metal pollution of water. J. Chem. Pharma. Res. 2015, 7, 700–705. [Google Scholar]
- Gautam, P.K.; Gautam, R.K.; Banerjee, S.; Chattopadhyaya, M.C.; Pandey, J.D. Heavy metals in the environment: Fate, transport, toxicity and remediation technologies. In Heavy Metals: Sources, Toxicity and Remediation Techniques; Nova Science Publishers: New York, NY, USA, 2016; pp. 101–130. [Google Scholar]
- Murad, M.W.; Pereira, J.J. Malaysia: Environmental Health Issues. In Encyclopedia of Environmental Health, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 4, pp. 194–210. [Google Scholar]
- Batool, F.; Akbar, J.; Iqbal, S.; Noreen, S.; Bukhari, S.N.A. Study of Isothermal, Kinetic, and Thermodynamic Parameters for Adsorption of Cadmium: An Overview of Linear and Nonlinear Approach and Error Analysis. Bioinorg. Chem. Appl. 2018, 2018, 3463724. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Ullah, S.S.; Faiz, P.; Leng, S. Synthesis, mechanism, and performance assessment of zero-valent iron for metal-contaminated water remediation: A review. Clean Soil Air Water 2020, 48, 2000080. [Google Scholar] [CrossRef]
- Segura, Y.; Martínez, F.; Melero, J.A.; Fierro, J.L.G. Zero valent iron (ZVI) mediated Fenton degradation of industrial wastewater: Treatment performance and characterization of final composites. Chem. Eng. J. 2015, 269, 298–305. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate-based adsorbent—A review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Morimoto, K.; Anraku, S.; Hoshino, J.; Yoneda, T.; Sato, T. Surface complexation reactions of inorganic anions on hydrotalcite-like compounds. J. Colloid Interface Sci. 2012, 384, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Yi, B.; Yuan, Q.; Wu, Y.; Wang, M.; Yan, S. Removal of methylene blue from aqueous solution by cattle manure-derived low temperature biochar. RSC Adv. 2018, 8, 19917–19929. [Google Scholar] [CrossRef] [Green Version]
- Helmenstine, A.M. What Adsorption Means in Chemistry. 2019. Available online: https://www.thoughtco.com/definition-of-adsorption-605820 (accessed on 3 February 2021).
- Ray, S.; Das, G. Chapter 12—Adsorption. In Process Equipment and Plant Design; Elsevier: Amsterdam, The Netherlands, 2020; pp. 351–384. [Google Scholar]
- Manzi, S.J.; Belardinelli, R.E.; Costanza, G.; Pereyra, V. Adsorption-Desorption Kinetics: A Review of a Classical Problem. arXiv 2008, arXiv:0802.2226. [Google Scholar]
- Gordon, E.B.; Andrea, L.F.; John, D.O. Mineral surfaces and bioavailability of heavy metals: A molecular-scale perspective. Proc. Natl. Acad. Sci. USA 1999, 96, 3388–3395. [Google Scholar]
- Aktar, J. Batch adsorption process in water treatment. In Intelligent Environmental Data Monitoring for Pollution Management; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 1–24. [Google Scholar]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Patterson, H.B.W. Chapter 2—Adsorption. In Bleaching and Purifying Fats and Oils, 2nd ed.; List, G.R., Ed.; AOCS Press: Champaign, IL, USA, 2009; pp. 53–67. [Google Scholar]
- Gao, B.; Gao, Y.; Li, Y. Preparation and chelation adsorption property of composite chelating material poly(amidoxime)/SiO2 towards heavy metal ions. Chem. Eng. J. 2010, 158, 542–549. [Google Scholar] [CrossRef]
- Zou, B.; Zhang, S.; Sun, P.; Ye, Z.; Zhao, Q.; Zhang, W.; Zhou, L. Preparation of a novel Poly-chloromethyl styrene chelating resin containing heterofluorenone pendant groups for the removal of Cu (II), Pb (II), and Ni (II) from wastewaters. Colloids Interface Sci. Commun. 2021, 40, 100349. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Orfanos, A.G.; Manariotis, I.D.; Tatarchuk, T.; Sellaoui, L.; Bonilla-Petriciolet, A.; Mittal, A.; Núñez-Delgado, A. Removal of caffeine, nicotine and amoxicillin from (waste)waters by various adsorbents. J. Environ. Manag. 2020, 261, 110236. [Google Scholar] [CrossRef] [PubMed]
- Eivazihollagh, A.; Svanedal, I.; Edlund, H.; Norgren, M. On chelating surfactants: Molecular perspectives and application prospects. J. Mol. Liq. 2019, 278, 688–705. [Google Scholar] [CrossRef]
- Eldridge, R.J. Chelating Ion Exchange Resins. Encycl. Sep. Sci. 2000, 2271–2279. [Google Scholar]
- Orf, G.M. Analytical Applications of Resins Containing Amide and Polyamine Functional Groups. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1977. Retrospective Theses and Dissertations. 6095. Available online: https://lib.dr.iastate.edu/rtd/6095 (accessed on 14 July 2021).
- Sahyoun, T.; Arrault, A.; Schneider, R. Amidoximes and oximes: Synthesis, structure, and their key role as No donors. Molecules 2019, 24, 2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.A. Methods for Hydroxamic Acid Synthesis. Curr. Org. Chem. 2019, 23, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Beccia, M.R. Hydroxamic Acids Interactions with Metals in Aqueous and Micellar Media: A Mechanistic Study of Complexation Reactions and Metallacrown Formation. Ph.D. Thesis, Università di Pisa, Pisa, Italy, 2014. Available online: https://tel.archives-ouvertes.fr/tel-00967266 (accessed on 21 June 2020).
- Bolotin, D.S.; Bokach, N.A.; Kukushkin, V.Y. Coordination chemistry and metal-involving reactions of amidoximes: Relevance to the chemistry of oximes and oxime ligands. Coord. Chem. Rev. 2016, 313, 62–93. [Google Scholar] [CrossRef] [Green Version]
- Arshad, S.; Amin, Z.; Puah, P.; Sarjadi, M.; Musta, B.; Rahman, M.L. Synthesis of Silica Gel Supported Amidoxime Ligand for Adsorption of Copper and Iron from Aqueous Media. Asian J. Chem. 2019, 31, 3035–3040. [Google Scholar] [CrossRef]
- Amran, M.B.; Panggabean, A.S.; Sulaeman, A.; Rusnadi, M. Preparation of a chelating resin and its application as a preconcentration system for determination of cadmium in river water by flow injection analysis. Int. J. Environ. Res. 2011, 5, 531–536. [Google Scholar]
- Eivazihollagh, A.; Tejera, J.; Svanedal, I.; Edlund, H.; Blanco, A.; Norgen, M. Removal of Cd2+, Zn2+, and Sr2+ by Ion Flotation, Using a Surface-Active Derivative of DPTA (C12-DTPA). Ind. Eng. Chem. Res. 2017, 56, 10605–10614. [Google Scholar] [CrossRef]
- Ojha, B.; Singh, A.K.; Adhikari, M.D.; Ramesh, A.; Das, G. 2-Alkylmalonic Acid: Amphiphilic Chelator and a Potent Inhibitor of Metalloenzyme. J. Phys. Chem. B. 2010, 114, 10835–10842. [Google Scholar] [CrossRef]
- Chua, S.F.; Nouri, A.; Ang, W.L.; Mahmoudi, E.; Mohammad, A.W.; Benamor, A.; Ba-Abbad, M. The emergence of multifunctional adsorbents and their role in environmental remediation. J. Environ. Chem. Eng. 2021, 9, 104793. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Tang, Z.; Huang, Q.; Niu, F.; Wang, X. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Rahman, M.L.; Fui, C.J.; Sarjadi, M.S.; Arshad, S.E.; Musta, B.; Abdullah, M.H.; Sarkar, S.M.; O’Reilly, E.J. Poly(amidoxime) ligand derived from waste palm fiber for the removal of heavy metals from electroplating wastewater. Environ. Sci. Pollut. Res. 2020, 27, 34541–34556. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.L.; Fui, C.J.; Ting, T.X.; Sarjadi, M.S.; Arshad, S.E.; Musta, B. Polymer ligands derived from jute fiber for heavy metal removal from electroplating wastewater. Polymers 2020, 12, 2521. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.L.; Wong, Z.J.; Sarjadi, M.S.; Soloi, S.; Arshad, S.E.; Bidin, K.; Musta, B. Heavy metals removal from electroplating wastewater by waste fiber-based poly(Amidoxime) ligand. Water 2021, 13, 1260. [Google Scholar] [CrossRef]
- Rahman, M.L.; Sarkar, S.M.; Yusoff, M.M.; Abdullah, M.H. Optical detection and efficient removal of transition metal ions from water using poly(hydroxamic acid) ligand. Sens. Actuators B Chem. 2017, 242, 595–608. [Google Scholar] [CrossRef]
- Rahman, M.L.; Wong, Z.J.; Sarjadi, M.S.; Abdullah, M.H.; Heffernan, M.A.; Sarkar, M.S.; O’Reilly, E. Poly(hydroxamic acid) ligand from palm-based waste materials for removal of heavy metals from electroplating wastewater. J. Appl. Polym. Sci. 2021, 138, 49671. [Google Scholar] [CrossRef]
- Rahman, M.L.; Wong, Z.J.; Sarjadi, M.S.; Joseph, C.G.; Arshad, S.E.; Musta, B.; Abdullah, M.H. Waste fiber-based poly(Hydroxamic acid) ligand for toxic metals removal from industrial wastewater. Polymers 2021, 13, 1486. [Google Scholar] [CrossRef]
- Tripathi, A.; Rawat Ranjan, M. Heavy Metal Removal from Wastewater Using Low Cost Adsorbents. J. Bioremed Biodeg. 2015, 6, 315. [Google Scholar] [CrossRef]
- Mi, H.; Yi, L.; Wu, Q.; Xia, J.; Zhang, B. Colloids and Surfaces A: Physicochemical and Engineering Aspects Preparation and optimization of a low-cost adsorbent for heavy metal ions from red mud using fraction factorial design and Box-Behnken response methodology. Physicochem. Eng. Asp. 2021, 627, 127198. [Google Scholar] [CrossRef]
- Park, J.H.; Eom, J.H.; Lee, S.L.; Hwang, S.W.; Kim, S.H.; Kang, S.W.; Yun, J.J.; Cho, J.S.; Lee, Y.H.; Seo, D.C. Exploration of the potential capacity of fly ash and bottom ash derived from wood pellet-based thermal power plant for heavy metal removal. Sci. Total Environ. 2020, 740, 140205. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Creamer, A.E.; He, F. Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: Batch and continuous flow tests. J. Hazard. Mater. 2017, 322, 172–181. [Google Scholar] [CrossRef]
- Lim, A.P.; Aris, A.Z. A review on economically adsorbents on heavy metals removal in water and wastewater. Rev. Environ. Sci. Biotechnol. 2014, 13, 163–181. [Google Scholar] [CrossRef]
- Yin, X.; Bai, J.; Tian, W.; Li, S.; Wang, J.; Wu, X.; Wang, Y.; Fan, F.; Huang, Q.; Qin, Z. Uranium sorption from saline lake brine by amidoximated silica. J. Radioanal. Nucl. Chem. 2017, 313, 113–121. [Google Scholar] [CrossRef]
- Wu, S.P.; Dai, X.Z.; Kan, J.R.; Shilong, F.D.; Zhu, M.Y. Fabrication of carboxymethyl chitosan–hemicellulose resin for adsorptive removal of heavy metals from wastewater. Chin. Chem. Lett. 2017, 28, 625–632. [Google Scholar] [CrossRef]
- Dev, V.V.; Baburaj, G.; Antony, S.; Arun, V.; Krishnan, K.A. Zwitterion-chitosan bed for the simultaneous immobilization of Zn(II), Cd(II), Pb(II) and Cu(II) from multi-metal aqueous systems. J. Clean. Prod. 2020, 255, 120309. [Google Scholar] [CrossRef]
- Chen, M.; Nong, S.; Zhao, Y.; Riaz, M.S.; Xiao, Y.; Molokeev, M.S.; Huang, F. Renewable P-type zeolite for superior absorption of heavy metals: Isotherms, kinetics, and mechanism. Sci. Total Environ. 2020, 726, 138535. [Google Scholar] [CrossRef] [PubMed]
- Abegunde, S.M.; Idowu, K.S.; Adejuwon, O.M.; Adeyemi-Adejolu, T. A review on the influence of chemical modification on the performance of adsorbents. Resour. Environ. Sustain. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Chmielewská, E. Chapter 4—Natural zeolite: Alternative adsorbent in purification or post-treatment of waters. In Modified Clay and Zeolite Nanocomposite Materials: Environmental and Pharmaceutical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–112. [Google Scholar]
- Sun, Z.; Wang, M.; Fan, J.; Zhou, Y.; Zhang, L. Regeneration performance of activated carbon for desulfurization. Appl. Sci. 2020, 10, 6107. [Google Scholar] [CrossRef]
- Wang, M.; Qu, R.; Sun, C.; Yin, P.; Chen, H. Dynamic adsorption behavior and mechanism of transition metal ions on silica gels functionalized with hydroxyl- or amino-terminated polyamines. Chem. Eng. J. 2013, 221, 264–274. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Najafi, M.; Yousefi, Y.; Rafati, A.A. Synthesis, characterization and adsorption studies of several heavy metal ions on amino-functionalized silica nano hollow sphere and silica gel. Sep. Purif. Technol. 2012, 85, 193–205. [Google Scholar] [CrossRef]
- Karbeka, M.; Nuryono; Suyanta. Coating of mercapto modified silica on iron sand magnetic material for Au(III) adsorption in aqueous solution. IOP Conf. Ser. Mater. Sci. Eng. 2020, 823, 012031. [Google Scholar] [CrossRef]
- Yuan, N.; Zhang, X.; Wang, L. The marriage of metal–organic frameworks and silica materials for advanced applications. Coord. Chem. Rev. 2020, 421, 213442. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Mirbagheri, A.; Ehteshami, M. Comparison of silica, activated carbon and zeolite adsorbents in the removal of ammonium, iron, COD, turbidity and phosphate pollutants, and investigating the effect of discharge on the removal of pollutants. Int. J. Humanit. Cult. Stud. 2016, 667–679. [Google Scholar]
- Abdulrazak, S.; Hussaini, K.; Sani, H.M. Evaluation of removal efficiency of heavy metals by low-cost activated carbon prepared from African palm fruit. Appl. Water Sci. 2016, 7, 3151–3155. [Google Scholar] [CrossRef] [Green Version]
- Ain, Q.U.; Farooq, M.U.; Jalees, M.I. Application of Magnetic Graphene Oxide for Water Purification: Heavy Metals Removal and Disinfection. J. Water Process Eng. 2020, 33, 101044. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, K.; Cai, J.; Hou, S.; Wang, Y.; Jiang, P.; Liang, M. Silica Oxide Encapsulated Natural Zeolite for High Efficiency Removal of Low Concentration Heavy Metals in Water. Colloids Surf. A Phys. Eng. Asp. 2018, 561, 388–394. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Plasma Polymer-Functionalized Silica Particles for Heavy Metals Removal. ACS Appl. Mater. Interface 2015, 7, 4265–4274. [Google Scholar] [CrossRef]
- Igberase, E.; Osifo, P. Equilibrium, kinetic, thermodynamic and desorption studies of cadmium and lead by polyaniline grafted cross-linked chitosan beads from aqueous solution. J. Ind. Eng. Chem. 2015, 26, 340–347. [Google Scholar] [CrossRef]


| Metal Ions | Sources | Harmful Effect | Allowed Limit (ppm) * |
|---|---|---|---|
| As(V) | Volcanic activity, industries, paints, drugs, dyes and textile, agriculture, smelting, mining | Severe arsenicosis, pigmentation problems, nausea, skin, and kidney cancer | 0.01 |
| Mo(II) | Industrialization, pesticides, catalysts, alloys, non-corrosive agents | Mineral imbalance, increment of serum ceruloplasmin, urinary copper excretion, gout-like symptoms | 0.07 |
| Zn(II) | Steel production plant, coal fire stations, galvanized metal pipes | Fever, vomit, nausea, cramp in stomach, diarrhea | 3.00 |
| Mn(II) | Mining, dumping sites, agriculture, fertilizers, soil | Nerve system failure, mutagenic and hepatic encephalopathy | 0.10 |
| Cd(II) | Electroplating plant, metal smelting, paints, batteries production, fertilizers, alloy industry | malfunction of renal, pulmonary troubles, bone cancer, high blood pressure, Itai-Itai disease, bone abnormalities | 0.01 |
| Co(II) | Metallurgy, mining, electroplating industry, paint manufacturing, nuclear power factory, tanning | Skeletal defects, diarrhea, hypotension pulmonary, paralyzed | 1.30 ** |
| Ni(II) | Nickel plating, alloys, production of batteries | Carcinogenic, losses of hair, skin toxicity | 0.10 ** |
| Cu(II) | Battery manufacturing, plumbing corrosion | Headache, depression, low IQ | 1.30 |
| Pb(II) | Plumbing fixture, cable cover, ceramic, batteries, paints, wielding, extraction of lead, glass | Liver failure, neurological system damage, gastrointestinal tract impairment, high blood pressure, infertility, arthralgia | 0.05 |
| Hg(II) | Volcanic activity, mining operation, tanning, electroplating industries | Minamata disease, cancer | 0.002 |
| Assessment | Water Pollution | References |
|---|---|---|
| Source of Pollution | Industrialization, solid and liquid waste discharge, septic tanks system, eutrophication, mining, bio-solids, acidification, agricultural field, landfills, oil, and gas salt-water pits, neglected well sites, brine disturbance, combustion, impoundment of water, hydrocarbons. | [16] |
| Type of Pollutant | Heavy metals, industrial wastes, WWTPs, brewery, milk manufacturing plants, suspended solids, pathogens, Pharmaceutical and Personal Cosmetic Care (PPCPs), Persistent Organic Pollutants (POPs), toxic and nontoxic substances, pathogenic parasites, bio-concentrated metals, crop wastes, fertilizers, pesticides, plastics, shipwrecks, paper mills. | [19] |
| Risks | Cholera, kidney and heart dysfunction, terrible blood circulation, nauseous, nervous system problems, aquatic ecosystem impairment, death, diarrhea, typhoid. | [16] |
| Remediation Method | Source prevention actions, nutrients monitoring and control, disinfection, thermal treatment, bioremediation, phytoremediation, sewer, and proper septic tank usage, strengthened sustainable water quality and environment policies, water treatment method. | [15] |
| Classification | Methods |
|---|---|
| Physical | Adsorption Ion exchange Nanofiltration Reverse osmosis membrane Solvent extraction Ultrafiltration |
| Chemical | Electric flocculation Electrodeposition Electrodialysis Electrolysis Ferrite precipitation Insoluble salt precipitation Neutralization precipitation |
| Biological | Bio-flocculation Bio-sorption Phytoremediation Bio-precipitation Biotransformation |
| Method | Benefits | Limitations | Reference |
|---|---|---|---|
| Adsorption | A wide range of adsorbent, excellent adsorption capacity, simple and low cost. | Difficult regeneration of adsorbent and sludge, different adsorption capacity for different type of adsorbent. | [6] |
| Microbial degradation | Short process time, economically safe and produce non-hazardous product. | Toxic metal hinders microbial activity and possibility of clogging of pumping and injection wells. | [15] |
| Membrane filtration | Higher removal efficiency, No pollution loads, Removal of different contaminants. | Capital and running cost are high, Operation and Maintenance requirement cost, Toxic waste as product, Membrane fouling. | [20] |
| Ion exchange | High metal recovery, fewer sludge volume and limited pH tolerance. | Costly and high on maintenance | [21] |
| Coagulation/Flocculation | Quick process, inexpensive, straightforward process and coagulating agents are easily accessible. | Produce waste, low efficiency removal and required extra process such as sedimentation and filtration | [23] |
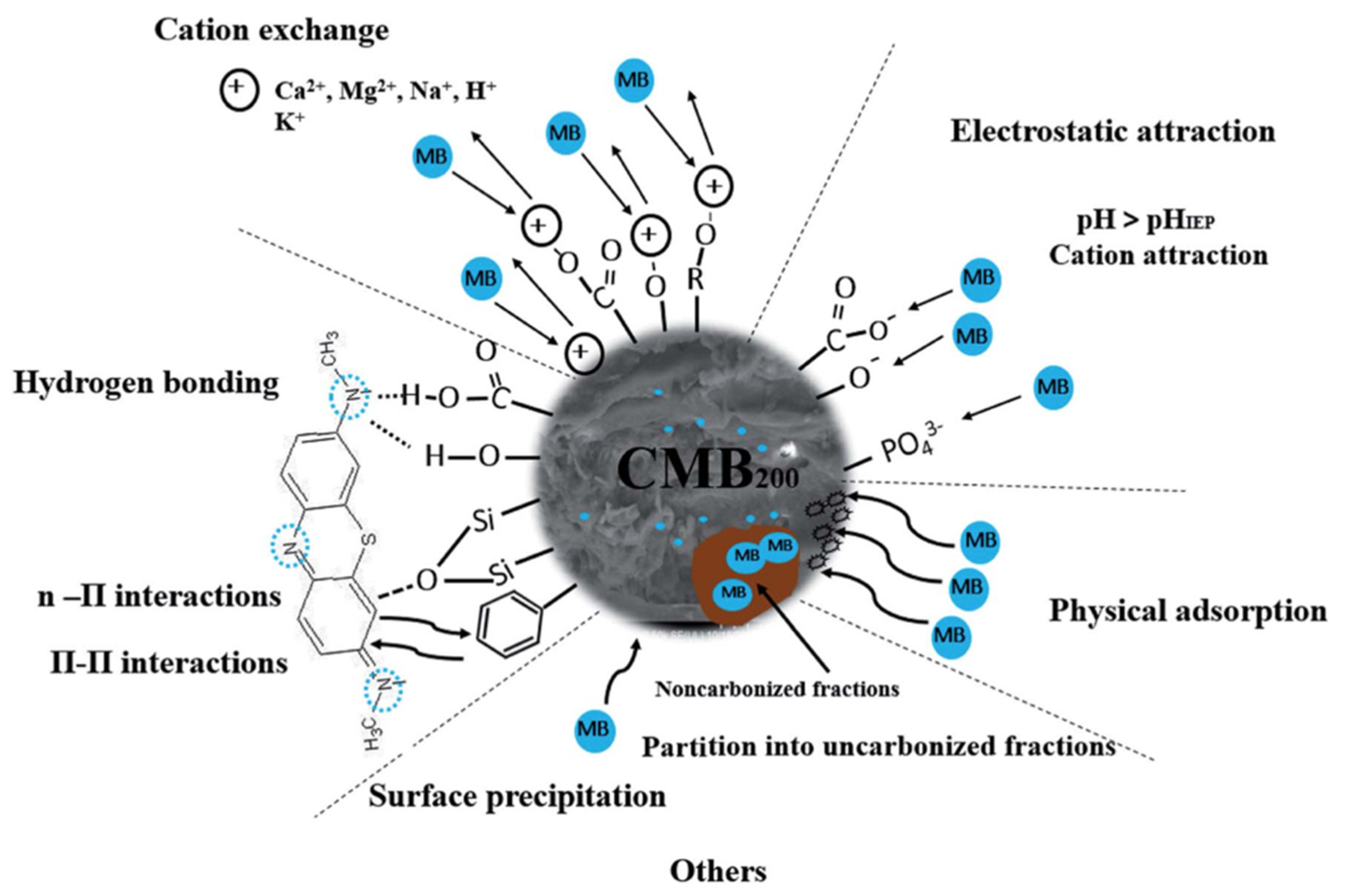
| Adsorption Mechanism | Mechanism Description and Illustration | References |
|---|---|---|
| Physical adsorption | Interaction between adsorbate and adsorbent surface through weak bonds, for instance weak van der Waals forces, hydrogen bonding or hydrophilic interactions.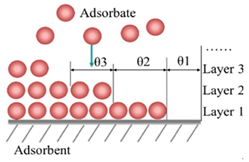 | [25] |
| Electrostatic interaction | Attraction between the ion and the surface of the opposite charge, e.g., positive charged ions with a negative surface of an adsorbent. | [26] |
| Ion exchange | A process involving the interchange of adsorbent and adsorbate with the matching ions charge. | [21] |
| Surface complexation | A process where direct bond between adsorbate and adsorbent surface occurs at the inner sphere complex, while the outer sphere complex interacts with adsorbent via electrostatic interaction while retaining the hydration sphere.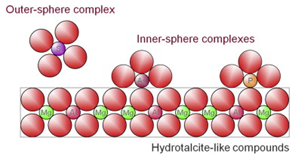 | [27] |
| Precipitation | Solid formation in solution or on a surface when the adsorbate ions interact on the adsorbent surface with a surface functional group due to a pH change. | [26] |
| Description | Chemisorption | Physisorption |
|---|---|---|
| Activation energy | Required | Not required |
| Temperature | High | Low |
| Enthalpy | 200–400 kJ/mol | 5–50 kJ/mol |
| Common adsorption formation | Unimolecular layer | Multimolecular layer |
| Factor Affecting Adsorption | Effect on Adsorption Process |
|---|---|
| pH | The presence of hydrogen ions (H+) and hydroxide ions (OH−) will react with the activated site of the adsorbent depending on the pH value. |
| pH at the potential of zero-point charge (pHzpc) | Assuming that the adsorbent’s surface is positive below the point of zero charges, it will allow negatively charged contaminants or pollutants to be adsorbed in batch processes. |
| Adsorbent dosage | Increase the active adsorption sites will be more effective in removing the contaminants or pollutants, although too much dosage will reduce the total uptake of pollutants (qe) |
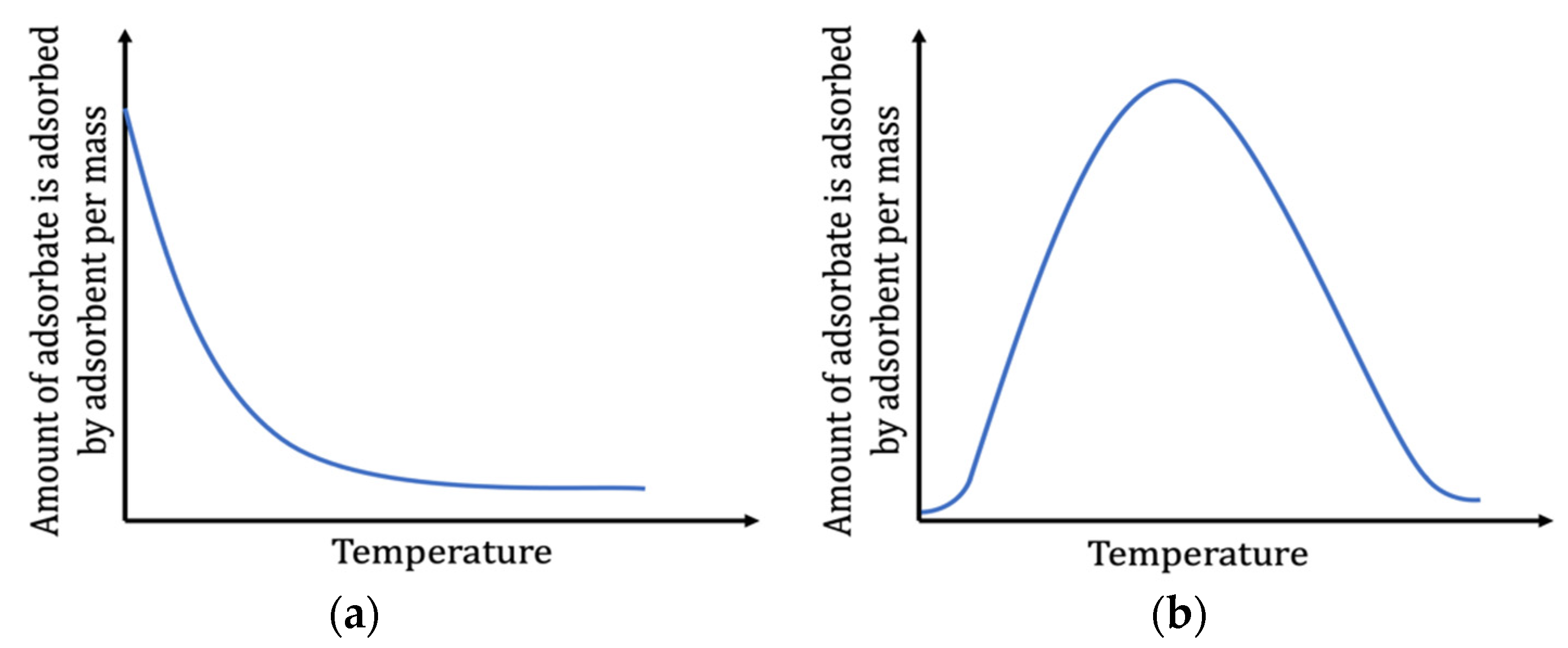
| Temperature | Higher temperature reduces the viscosity, which helps the mobility of contaminants from the bulk solution to the surface of the substance. |
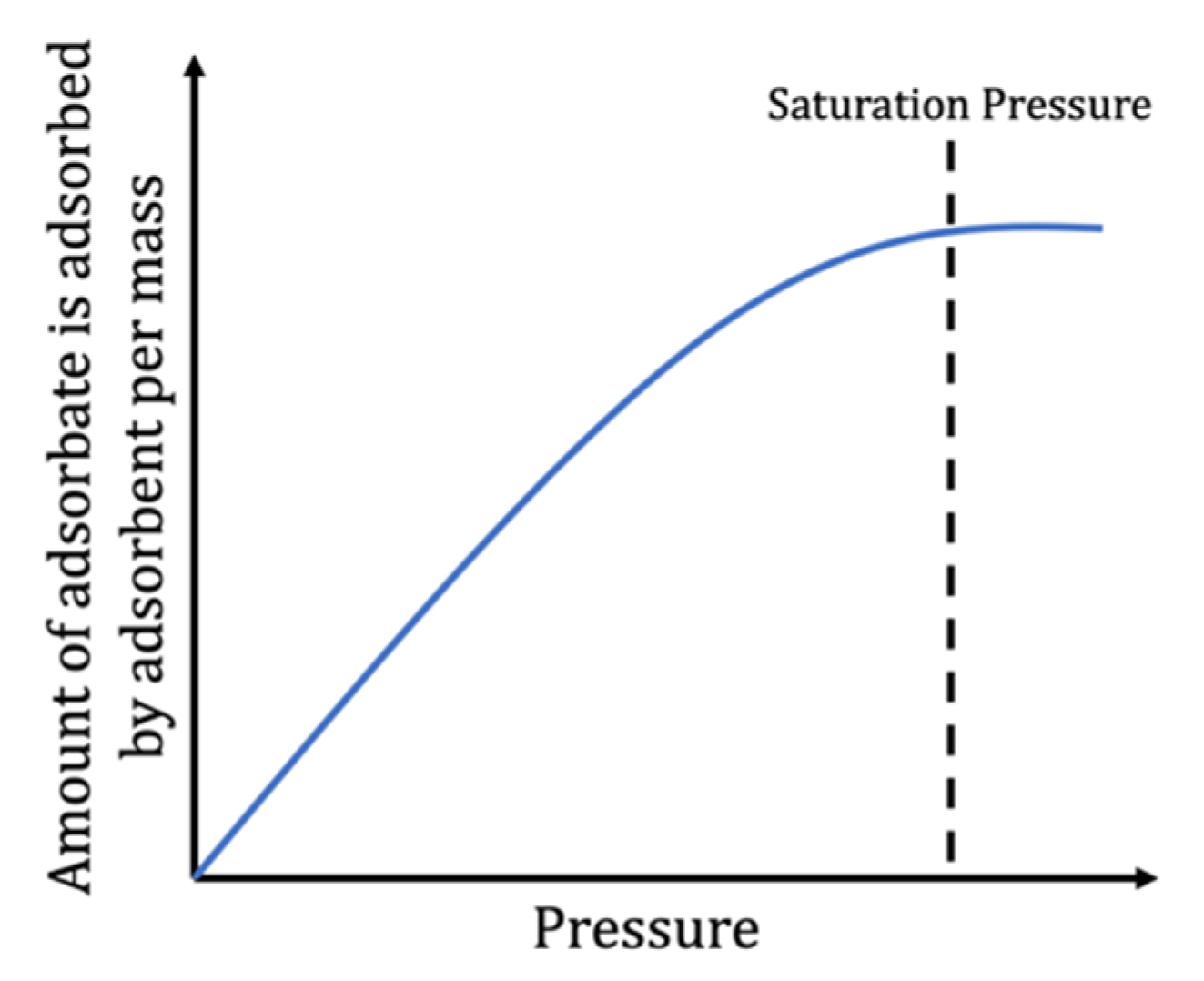
| Pressure | Intensify the adsorption process up to a higher level until the process reaches equilibrium. |
| Surface Area | Smaller particles have bigger surface area compared to the larger particles, allowing greater adsorption to occur. |
| Co-existing ions | Lesser co-existing ions in the solution will have a better adsorption process. |
| Application | Purpose | Method | Chelating Resins | Reference |
|---|---|---|---|---|
| Pollution Control | To remove any metal ions contaminant that degrades the environment.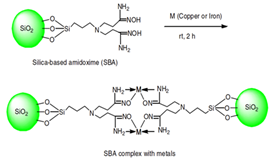 | Adsorption | Silica supported amidoxime ligand | [46] |
| Pre-concentration system | To enhance the detection of trace metal ions.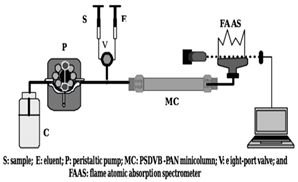 | Flow Injection Analysis | PSDVB-PAN | [47] |
| Removal of metal ions | To discharge non-surface-active ions from aqueous solutions by adding a surfactant followed by air flotation and assortment of formed foam. | Ion Flotation | Surface-Active Derivative of C12- DTPA | [48] |
| Biology processes | Act as an inhibitor for enzymes that contains metals, known as metalloenzymes. | Extraction | 2-alkylmalonic acid amphiphile | [49] |
| Adsorbent Classification | Examples |
|---|---|
| Polymeric based |
|
| Industrial By-product |
|
| Natural Minerals based |
|
| Carbon Nanomaterial based |
|
| Adsorbent | Target Metal | Optimum pH | Adsorption Capacity (mg g−1) | Kinetics Model | Isotherm Model * | Reference |
|---|---|---|---|---|---|---|
| Poly(amidoxime)-palm cellulose | Cu | 6 | 260.0 | Pseudo-first order | F | [52] |
| Fe | 6 | 210.0 | Pseudo-first order | F | ||
| Co | 6 | 168.0 | Pseudo-first order | F | ||
| Ni | 6 | 172.0 | Pseudo-first order | F | ||
| Pb | 6 | 272.0 | Pseudo-first order | F | ||
| Poly(amidoxime)-jute cellulose | Cu | 6 | 310.0 | Pseudo-second order | F | [53] |
| Co | 6 | 295.0 | Pseudo-second order | F | ||
| Cr | 6 | 227.0 | Pseudo-second order | F | ||
| Ni | 6 | 175.0 | Pseudo-second order | F | ||
| Poly(amidoxime)-waste cellulose | Cu | 6 | 298.4 | Pseudo second order | L | [54] |
| Co | 6 | 289.6 | Pseudo-second order | L | ||
| Cr | 6 | 217.0 | Pseudo-second order | L | ||
| Ni | 6 | 168.7 | Pseudo-second order | L | ||
| Poly(hydroxamic acid)-kenaf cellulose | Cu | 6 | 305.3 | Pseudo-second order | L | [55] |
| Fe | 6 | 275.6 | Pseudo-second order | L | ||
| Mn | 6 | 258.5 | Pseudo-second order | L | ||
| Co | 6 | 256.6 | Pseudo-second order | L | ||
| Cr | 6 | 254.3 | Pseudo-second order | L | ||
| Ni | 6 | 198.5 | Pseudo-second order | L | ||
| Zn | 6 | 190.1 | Pseudo-second order | L | ||
| Poly(hydroxamic acid)-palm cellulose | Cu | 6 | 325.0 | Pseudo-first order | F | [56] |
| Fe | 6 | 220.0 | Pseudo-first order | F | ||
| Pb | 6 | 300.0 | Pseudo-first order | F | ||
| Poly(hydroxamic acid)-jute cellulose | Cu | 6 | 352.0 | Pseudo-first order | F | [53] |
| Co | 6 | 318.0 | Pseudo-first order | F | ||
| Cr | 6 | 230.0 | Pseudo-first order | F | ||
| Ni | 6 | 188.0 | Pseudo-first order | F | ||
| Poly(hydroxamic acid)-waste fiber | Cu | 6 | 346.7 | Pseudo-second order | L | [57] |
| Co | 6 | 315.0 | Pseudo-second order | L | ||
| Cr | 6 | 227.6 | Pseudo-second order | L | ||
| Ni | 6 | 181.4 | Pseudo-second order | L |
| Adsorbent | Target Metal | Optimum pH | Adsorption Capacity (mg g−1) | Kinetics Model | Isotherm Model * | Reference |
|---|---|---|---|---|---|---|
| Red mud | Pb | 5 | 128.53 | Pseudo-second order | N/A | [59] |
| Zn | 5 | 35.70 | Pseudo-second order | N/A | ||
| Fly ash | Pb | 6 | 194.70 | Pseudo-second order | L | [60] |
| Cu | 6 | 151.40 | Pseudo-second order | L | ||
| Cd | 6 | 143.10 | Pseudo-second order | L | ||
| Zn | 6 | 92.60 | Pseudo-second order | L | ||
| Bottom ash | Pb | 5-6 | 53.20 | Pseudo-second order | L | [60] |
| Cu | 5-6 | 32.40 | Pseudo-second order | L | ||
| Cd | 5-6 | 23.60 | Pseudo-second order | L | ||
| Zn | 5-6 | 15.80 | Pseudo-second order | L | ||
| Biochar supported zero-valent iron nanocomposite | As | 4.1 | 124.5 | Both PFO and PSO | F and L | [61] |
| Adsorbent | Target Metal | Optimum pH | Adsorption Capacity (mg g−1) | Kinetics Model | Isotherm Model * | Reference |
|---|---|---|---|---|---|---|
| Carboxymethyl chitosan–hemicellulose | Cu | 6 | 362.30 | Pseudo-second order | L | [64] |
| Cr | 4 | 909.10 | Pseudo-second order | L | ||
| Hg | 4 | 333.30 | Pseudo-second order | L | ||
| Ni | 4 | 42.00 | Pseudo-second order | L | ||
| Cd | 4 | 28.20 | Pseudo-second order | L | ||
| Mn | 4 | 49.00 | Pseudo-second order | L | ||
| Poly(amidoxime)-silica | Cu | 6 | 172.00 | Pseudo-first order | F | [46] |
| Fe | 6 | 168.00 | Pseudo-first order | F | ||
| Carboxylate functionalized-chitosan co-polymer | Pb | 6 | 127.91 | Pseudo-second order | L | [65] |
| Cu | 6 | 123.50 | Pseudo-second order | L | ||
| Cd | 6 | 108.42 | Pseudo-second order | L | ||
| Zn | 6 | 92.27 | Pseudo-second order | L | ||
| Synthetic NASO Zeolite (Na6Al6Si10O32.12H2O) | Cd | 5 | 649.00 | Pseudo-second order | L | [66] |
| Pb | 5 | 210.00 | Pseudo-second order | L |
| Pollutant | Initial Pollutant Concentration (mg/L) | Final Concentration with Silica (mg/L) | Final Concentration with Zeolite (mg/L) | Final Concentration with Activated Carbon (mg/L) | Permissible Level in Drinking Water (mg/L) |
|---|---|---|---|---|---|
| Ammonium | 5.50 | 4.70 | 1.50 | 3.50 | 1.50 |
| Iron | 0.55 | 0.10 | 0.50 | 0.35 | 0.3 |
| Phosphate | 4.0 | 2.80 | 1.20 | 2.50 | N/A |
| COD | 200 | 70.0 | 180 | 21.0 | 0 |
| Turbidity | 100 * | 8.1 * | N/A | 9.7 * | 5 * |
| Adsorbent | Target Metal | pH | Initial Metal Concentration (mg/L) | Contact Time (min) | Adsorption Capacity (mg/g) | Removal Percentage (%) | Reference |
|---|---|---|---|---|---|---|---|
| Activated carbon from African palm fruit | Cd | 8 | 1820.00 | 60 | N/A | 99.23 | [76] |
| Cu | 3 | 1520.00 | 60 | N/A | 96.71 | ||
| Ni | 8 | 3240.00 | 60 | N/A | 95.34 | ||
| Pb | 3 | 2620.00 | 60 | N/A | 97.75 | ||
| Magnetic graphene oxide | Pb | 5 | 60.00 | 25 | 200.00 | 89.61 | [77] |
| Cr | 6 | 60.00 | 35 | 24.330 | 92.03 | ||
| Cu | 6 | 60.00 | 25 | 62.89 | 92.43 | ||
| Zn | 7 | 60.00 | 35 | 63.69 | 90.38 | ||
| Ni | 8 | 60.00 | 25 | 51.02 | 92.23 | ||
| Silica oxide encapsulated natural zeolite | Pb | N/A | 10.00 | 30 | 186.00 | 99.30 | [78] |
| Cu | N/A | 10.00 | 480 | 10.30 | 98.90 | ||
| Cd | N/A | 10.00 | 60 | 12.30 | 98.30 | ||
| Zn | N/A | 10.00 | 60 | 9.00 | 97.10 | ||
| Mn | N/A | 10.00 | 30 | 4.20 | 54.00 | ||
| Plasma polymer functionalized silica | Cu | 5.5 | 15.00 | 60 | 25.00 | >96.70 | [79] |
| Zn | 5.5 | 15.00 | 60 | 27.40 | >96.70 | ||
| Polyaniline grafted cross-linked chitosan beads | Cd | 6 | 40.00 | 60 | 145.00 | 99.60 | [80] |
| Pb | 5 | 40.00 | 60 | 114.00 | 99.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. https://doi.org/10.3390/w13192659
Zaimee MZA, Sarjadi MS, Rahman ML. Heavy Metals Removal from Water by Efficient Adsorbents. Water. 2021; 13(19):2659. https://doi.org/10.3390/w13192659
Chicago/Turabian StyleZaimee, Muhammad Zaim Anaqi, Mohd Sani Sarjadi, and Md Lutfor Rahman. 2021. "Heavy Metals Removal from Water by Efficient Adsorbents" Water 13, no. 19: 2659. https://doi.org/10.3390/w13192659






